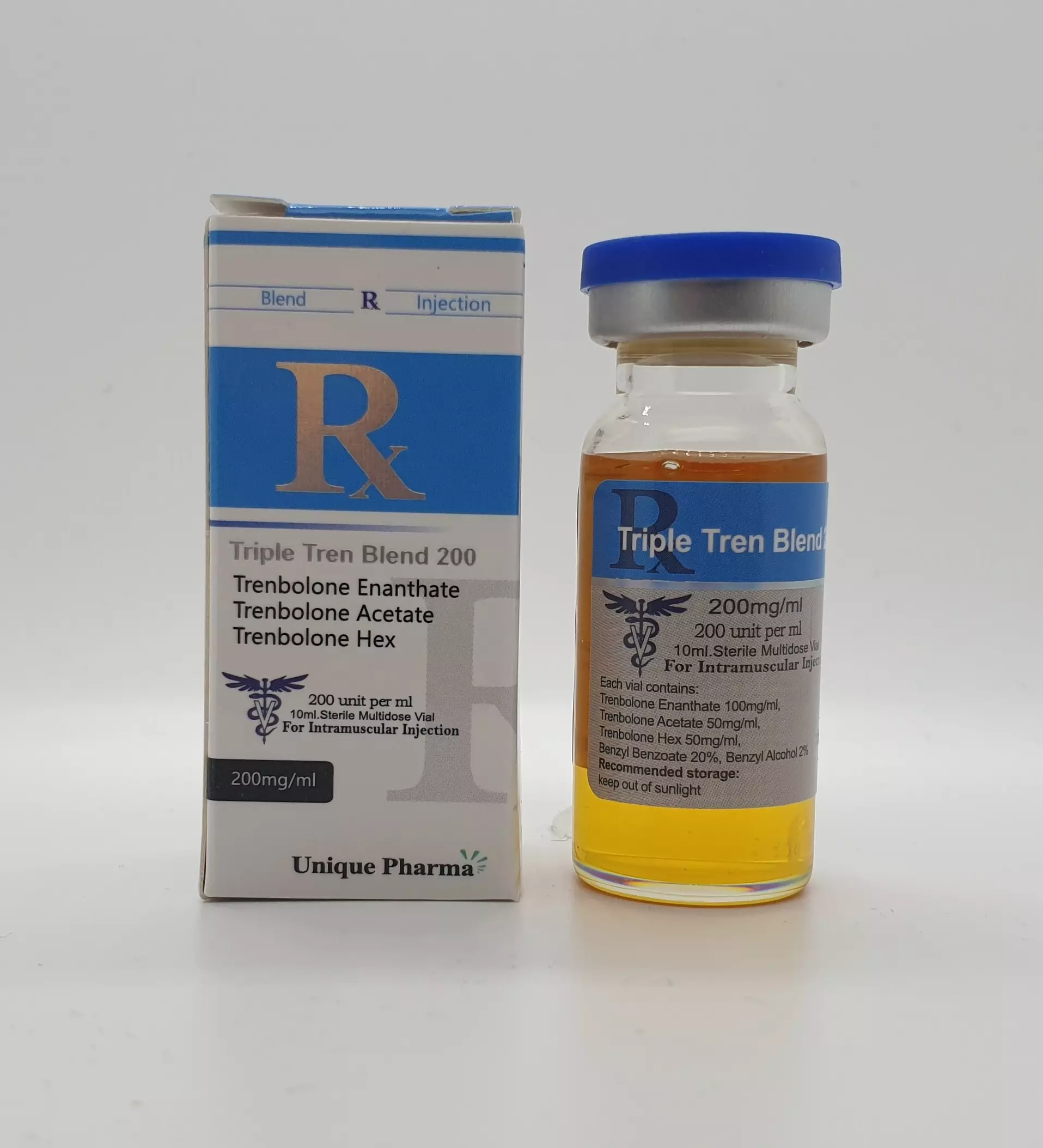

Tri-Tren 200 mg/ml (UP)
€ 55,00
Op voorraadBuy premium Tri-Tren (Trenbolone Blend) from Unique Pharma. Lab-tested, fast shipping, competitive prices.
Acne
Yes
Halfwaardetijd
10 Days
Dosering
200-400mg Weekly
Detectietijd
120-150 Days
Aromatisering
No
Water Retentie
No
Hepatotoxiciteit
Yes
HBR
Yes
Product Informatie
Over Tri-Tren 200 mg/ml (UP)
1. Description — clinical summary
"Tri Tren 200 mg" (active: described by the label as “tri‑tren”) is not a recognized international nonproprietary drug name (INN) or an approved pharmaceutical compound in major regulatory authorities. The name suggests a product related to trenbolone-class anabolic–androgenic steroids (AAS) or a trenbolone-containing blend, which are compounds primarily used in veterinary contexts (e.g., growth promoters) or circulated in non‑medical markets for performance enhancement.
Important clinical points
- There are no established, approved medical indications, dosing guidelines, or regulatory approvals for “tri‑tren” as a prescription medicine in humans.
- Use in humans is typically off‑label/illicit and associated with substantial risks.
- Information below summarizes likely pharmacology, expected risks, and prudent medical considerations for clinicians and patients encountering this substance.
If you have this product or a patient who has used it, seek guidance from a licensed clinician or local toxicology/poison control service; do not rely on non‑verified product labeling.
2. How does tri‑tren work? — mechanism of action
Based on the likely relationship to trenbolone-like molecules, the expected mechanism is consistent with potent androgen receptor (AR) agonists:
- Binds to androgen receptors in muscle, bone and other tissues, activating androgenic and anabolic signaling pathways that increase protein synthesis, nitrogen retention, and muscle cell hypertrophy.
- Strong androgenic activity: promotes anabolic effects more potently than testosterone in many assays.
- Does not significantly aromatize to estrogen (trenbolone itself has little to no aromatization), so estrogenic side effects (gynaecomastia from aromatization) are less common, but other androgenic adverse effects remain.
- Suppresses the hypothalamic–pituitary–gonadal (HPG) axis: exogenous AR agonists reduce gonadotropin release, causing decreased endogenous testosterone production, testicular atrophy, and infertility during and potentially after exposure.
- Metabolic and systemic effects: can adversely affect lipid profiles (lower HDL, raise LDL), increase hematocrit (polycythemia), and influence fluid balance and blood pressure. Some trenbolone derivatives have been associated with neuropsychiatric effects (mood changes, aggression, insomnia).
Exact pharmacokinetics (absorption, half‑life, metabolism) depend on the chemical structure, route of administration (oral vs injectable; esterified forms), and formulation. These details are often absent or unreliable for non‑regulated products.
3. Dosage — medical and varying usage guidelines
Regulatory/clinical status
- There are no approved clinical dosing regimens for “tri‑tren” in humans. No standardized, evidence‑based medical dosages exist.
- Any use in humans outside an authorized clinical trial should be considered off‑label and carries significant medical and legal risk.
Medical guidance for clinicians
- If exposure has occurred, management is supportive and guided by symptoms and laboratory findings. There is no antidote.
- Evaluate and monitor: baseline and follow‑up measurements (see section on monitoring below).
- For patients seeking to discontinue use: discuss expected timeline of HPG suppression and consider referral to endocrinology for evaluation of hypogonadism and potential need for hormone replacement or assisted recovery strategies.
Harm‑reduction and safety principles (non‑prescriptive)
- Because there is no safe, approved dosing standard for non‑medical use, avoid providing specific regimens. Higher and more frequent dosing predictably raises the risk of severe adverse effects (cardiovascular, hepatic, psychiatric, endocrine).
- If an individual insists on continuing or has been exposed, prioritize medical evaluation, baseline testing, and regular monitoring rather than advising dosing strategies.
- For injectable preparations, harm‑reduction measures include sterile technique, single‑use needles, safe disposal, and avoidance of intravascular injection — these reduce infectious and local complications but do not mitigate systemic effects.
Bottom line: do not use unapproved anabolic agents. If exposure occurs, seek medical evaluation rather than attempting to self‑dose or self‑manage.
4. Side effects — common and rare adverse effects
Common/expected adverse effects (based on trenbolone/AAS class)
- Endocrine: suppression of endogenous testosterone, decreased sperm production and fertility, testicular atrophy, menstrual irregularities in women.
- Androgenic/virilization (especially in females): deepening of voice, hirsutism/acne, male pattern baldness, clitoral enlargement. Some changes may be irreversible.
- Cardiovascular/metabolic: adverse lipid changes (↓ HDL, ↑ LDL), hypertension, increased risk of thrombosis, long‑term increased cardiovascular risk.
- Hematologic: increased hematocrit/hemoglobin (polycythemia) with risk of hyperviscosity.
- Psychiatric/neurologic: mood swings, increased aggression/irritability, anxiety, depression, insomnia. Some users report severe mood effects or “tren cough” (transient cough following injection reported anecdotally with some trenbolone products).
- Hepatic: varying risk depending on chemical modification. 17‑alpha‑alkylated oral AAS have clear hepatotoxicity; injectable oil‑based steroid esters carry less direct hepatotoxicity yet may still affect liver tests.
- Renal: possible adverse effects on renal function, particularly with prolonged high‑dose use or in combination with other agents.
- Local: injection‑site pain, abscess, cellulitis if non‑sterile technique is used.
Less common/serious/rare adverse effects
- Acute coronary events, stroke, or other major thrombotic events, especially in presence of other risk factors.
- Severe liver injury or cholestatic jaundice (more typical of certain oral AAS).
- Severe psychiatric events including mania or suicidal ideation.
- Permanent virilization in women.
- Serious infections (bacterial endocarditis, systemic infections) from non‑sterile injections.
- Persistent hypogonadism after discontinuation requiring long‑term androgen replacement.
Contraindications and cautions
- Known prostate or breast cancer (androgens can stimulate growth).
- Pregnancy and breastfeeding (androgens cause fetal harm — virilization).
- Uncontrolled cardiovascular disease, severe dyslipidemia, active liver disease, established polycythemia.
- Pediatrics and adolescents: use will impair growth and sexual maturation.
If severe symptoms occur (chest pain, sudden shortness of breath, signs of stroke, jaundice, severe mood changes, high fever, sepsis), seek emergency medical care.
Monitoring recommendations for clinicians (if patient exposed)
- Baseline and periodic: lipid panel, liver function tests (ALT/AST, bilirubin), CBC including hematocrit/hemoglobin, renal function (creatinine/BUN), testosterone/LH/FSH if endocrine issues suspected, and PSA in older men. Frequency depends on exposure and clinical context, but baseline then periodic (e.g., 4–12 week intervals) while exposure continues is common in clinical practice. Monitor blood pressure and mental health status.
5. Storage — how to store it
Because “Tri Tren 200 mg” is not a regulated product, storage instructions may vary by formulation. Follow these general pharmaceutical storage principles:
- Read the manufacturer label if available. If labeling is absent or unreliable, treat the product cautiously and consult a pharmacist or clinician.
- Injectable (oil‑based vials/ampoules): store at controlled room temperature (typical guidance 15–25 °C / 59–77 °F) unless the label specifies refrigeration. Avoid freezing. Protect from light and heat. Do not use if the solution is cloudy (if originally clear), contains particulate matter, or if the vial is compromised.
- Oral tablets/capsules: store in original container, at room temperature, away from excess heat and moisture (avoid bathroom), out of reach of children. Typical ambient storage 15–25 °C (59–77 °F) unless label specifies otherwise.
- Keep all pharmaceutical products in child‑resistant containers and away from pets.
- Do not use beyond expiry date. Dispose of needles and sharps in an approved sharps container. Dispose of unused or suspicious product per local hazardous/controlled medication disposal guidance.
Additional safety notes
- Because the composition of non‑regulated products may be unknown or contaminated, storage cannot mitigate risks from impurities or mislabeling. If the product looks, smells, or behaves unexpectedly, do not use it.
- If a patient brings such a product, clinicians should consider sending a sample for laboratory analysis only within the appropriate legal and institutional frameworks.
Closing and clinical recommendation
- “Tri Tren 200 mg” and similar trenbolone‑related products lack regulatory approval for human therapeutic use and are associated with significant short‑ and long‑term health risks.
- Clinicians: evaluate exposures medically, obtain baseline labs, counsel on cessation, monitor for endocrine and cardiometabolic effects, and treat complications. Consider consultation with endocrinology, cardiology, hepatology, psychiatry, or addiction medicine as indicated.
- Patients: avoid use of unapproved anabolic agents. If exposed or symptomatic, seek prompt medical evaluation. For acute poisoning or severe reaction, contact emergency services or local poison control.
If you want, I can: (a) provide a concise checklist for baseline monitoring and follow‑up labs, (b) outline emergency red flags to give to patients, or (c) draft language you can use to counsel a patient who has used this product.
Dosering
Aanbevolen
200-400mg Weekly
Halfwaardetijd
10 Days
Voordelen
- Laboratorium getest op zuiverheid
- Farmaceutische kwaliteit gegarandeerd
- Discrete en veilige verzending
- Uitstekende klantenservice
- Snelle levering in heel Europa
Gratis verzending
Gratis verzending bij bestellingen boven de €200.
Leveringsgarantie
Gratis herverzending als uw bestelling niet aankomt.
Snelle levering
Verzending binnen 24u. Levering 48-72u in NL & BE.
Bitcoin Betaling
Betaal veilig en blijf volledig anoniem met Bitcoin.
Veilig afrekenen
Beveiligde SSL-verbinding voor alle transacties.
Authentiek
Originaliteitscontrole voor al onze producten.