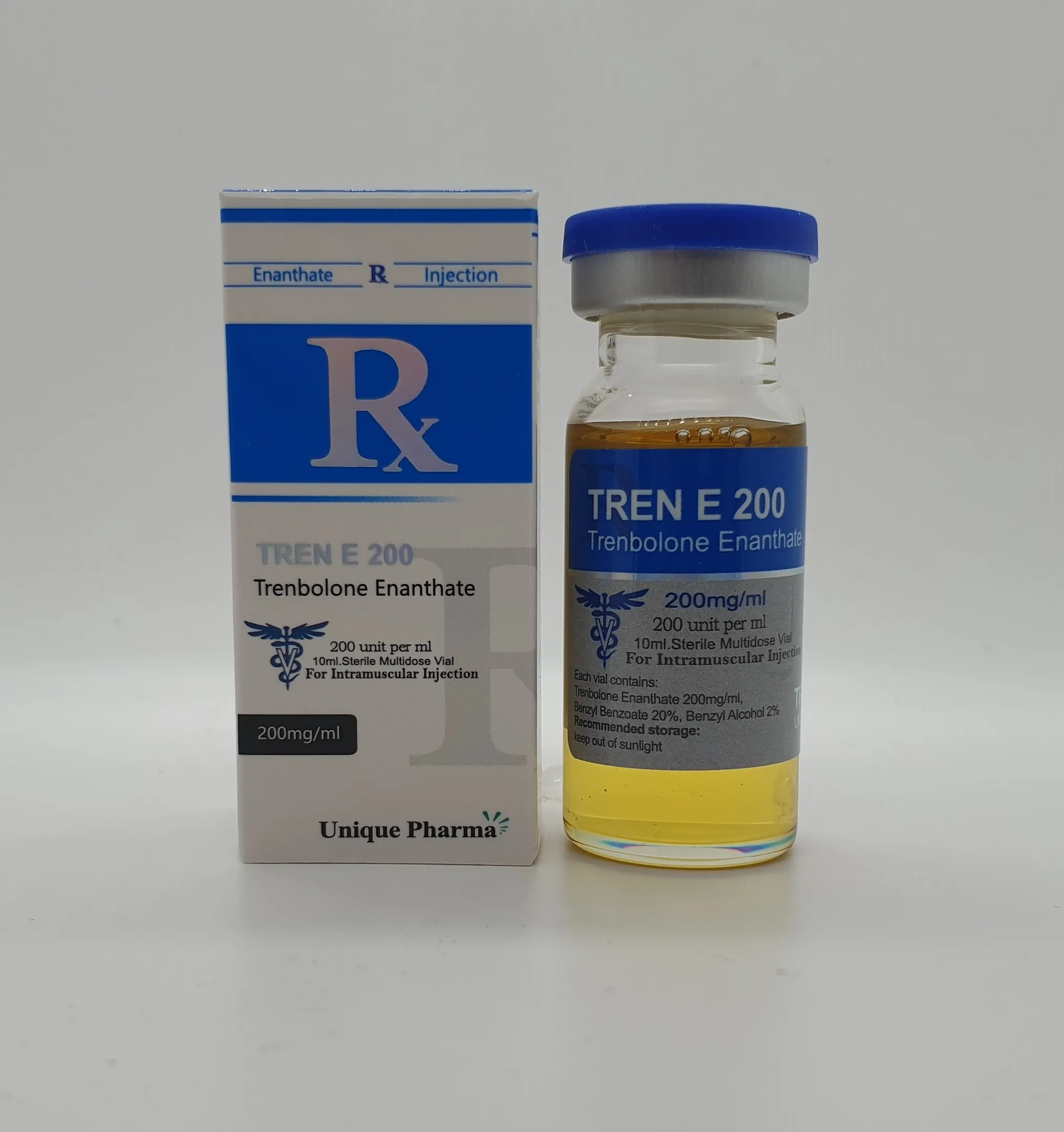
UNIQUE PHARMA QUALITY: Premium pharmaceutical grade Trenbolone Enanthate (Tren E) manufactured under strict GMP conditions with 99.8% purity verification.
Tren E from Unique Pharma represents our commitment to delivering exceptional quality performance enhancement compounds. Each batch undergoes rigorous testing to ensure consistent potency and purity standards.
Key Characteristics of Tren E
This injectable compound is administered via intramuscular injection and remains active in your system for approximately 10 Days. Notable features include:
- Pharmaceutical grade manufacturing
- Batch-tested for purity and potency
- Consistent dosing per unit
- Optimal bioavailability
Primary Benefits:
- Enhanced performance and recovery
- Quality-assured formulation
- Reliable and consistent results
- Professional-grade compound
Mechanism of Action
Trenbolone Enanthate works by interacting with androgen receptors in muscle tissue, promoting protein synthesis and nitrogen retention. This creates an optimal environment for muscle development and recovery. The compound's unique molecular structure provides specific benefits that distinguish it from other options in its class.
Usage Guidelines
Unique Pharma Tren E is suitable for experienced users who understand proper cycling protocols. Always consult with a healthcare professional before beginning any supplementation regimen. Proper post-cycle therapy should be considered based on individual needs and cycle duration.
Recommended Applications
This compound is commonly incorporated into both bulking and cutting protocols depending on the user's specific goals. Its versatility makes it a popular choice among athletes and bodybuilders seeking reliable results.
Potential Considerations
As with any performance compound, users should be aware of potential effects and monitor their response accordingly. Regular health monitoring is recommended during use. Individual responses may vary based on genetics, diet, training, and other factors.
Quality Assurance
Every Unique Pharma product undergoes comprehensive quality control including:
- Raw material verification
- In-process testing
- Final product analysis
- Stability testing
Warning: Keep out of reach of children. For adults only. Not intended for use by individuals under 18 years of age.
Related products
Other Unique Pharma products
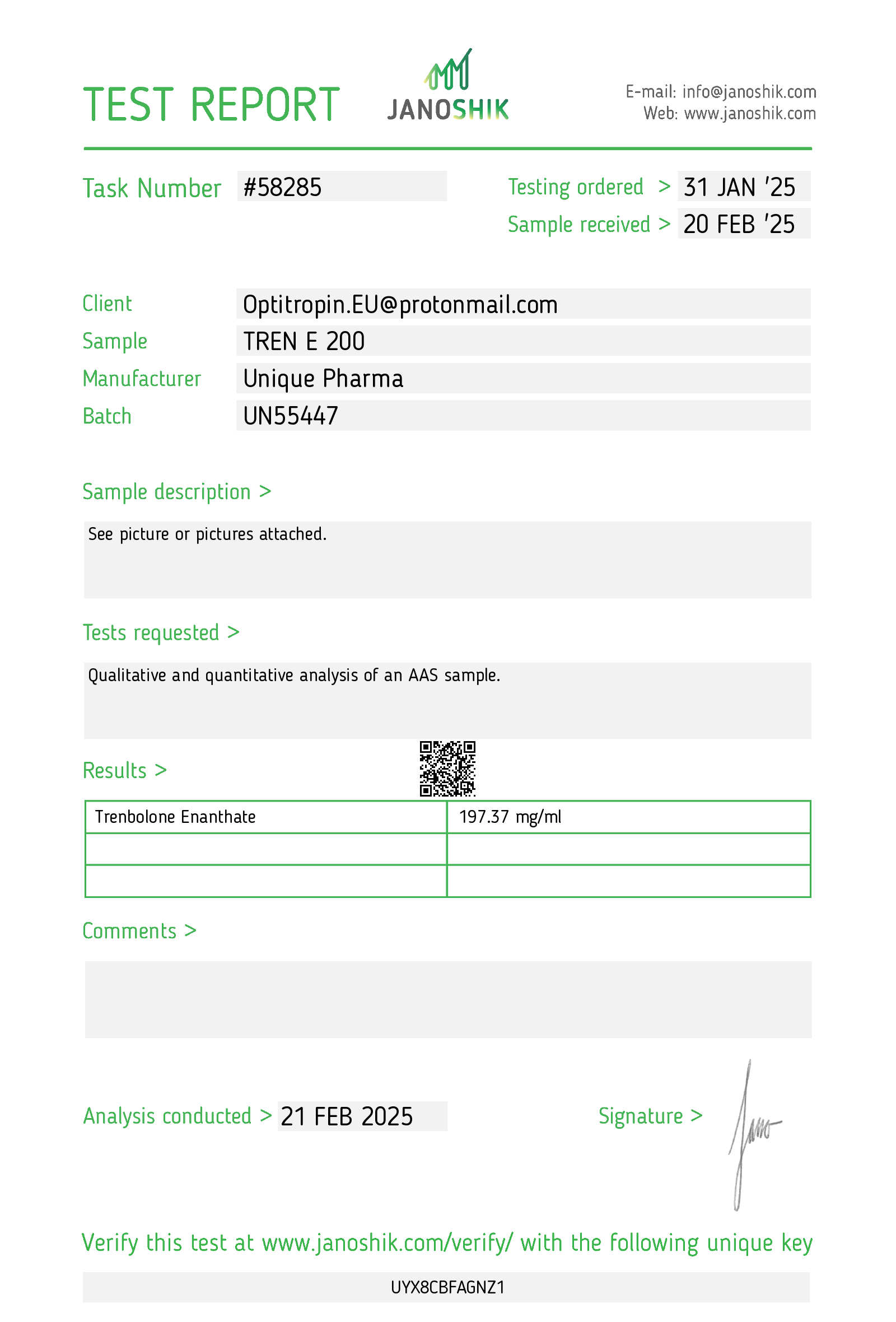
1. Description — clinical summary
Tren E 200 mg/mL refers to an injectable formulation containing trenbolone enanthate at a concentration of 200 mg per milliliter. Trenbolone is a potent synthetic anabolic–androgenic steroid (AAS) originally developed for veterinary use (to promote muscle/weight gain in livestock). The enanthate ester lengthens the compound’s action compared with the undecanoate/acetate esters, producing a relatively long duration of effect after intramuscular injection.
Important regulatory note: trenbolone is not approved for clinical use in humans in many countries. Products labeled “Tren E 200 mg/mL” are commonly encountered in veterinary medicine or the unregulated market and are frequently used illicitly for physique- or performance-enhancement. Legal status varies by country; possession or distribution without appropriate authority may be illegal.
Clinical context: because trenbolone is not an approved human medicine in most jurisdictions, there is no recognized, evidence-based medical dosing regimen for human therapy.
2. How does trenbolone-enanthate work? — mechanism of action
- Androgen receptor (AR) agonist: trenbolone binds the AR with high affinity. AR activation produces anabolic effects (increased protein synthesis, muscle growth) and androgenic effects (development/maintenance of male secondary sex characteristics).
- Potent anabolic and androgenic effects: trenbolone is significantly more potent than testosterone on a milligram-for-milligram basis in both anabolic and androgenic activity.
- Non-aromatizing but progestogenic: trenbolone does not convert to estradiol (it does not aromatize), so classical estrogen-driven side effects (water retention, marked gynecomastia from aromatization) are generally reduced compared with aromatizable AAS. However, trenbolone has intrinsic progestogenic activity at the progesterone receptor, which can still contribute to gynecomastia in some users and other hormonal effects.
- Effects on metabolism and hematology: trenbolone increases nitrogen retention, protein synthesis, and may increase insulin-like growth factor 1 (IGF‑1) activity locally in muscle. It also stimulates erythropoiesis (increased red blood cell mass), which can increase hematocrit and viscosity.
- Interactions with other endocrine systems: trenbolone suppresses the hypothalamic–pituitary–gonadal (HPG) axis, decreasing endogenous testosterone production and spermatogenesis. It may interact with glucocorticoid signaling, reducing catabolic cortisol effects.
3. Dosage — medical and varying-usage guidance
- No approved human medical dose: Trenbolone enanthate is generally not approved for human therapeutic use; therefore, no clinically validated or recommended human dosing exists. Any use outside an approved veterinary indication is off-label and often unlawful.
- Pharmacokinetic note: the enanthate ester confers a relatively long duration of action compared with shorter esters; plasma concentrations decline over days to a couple of weeks after a single injection. This influences dosing frequency in contexts where it is used, but pharmacokinetic parameters vary by formulation and individual.
- Harm-minimization and monitoring recommendations (for clinicians caring for patients who report exposure or use):
- Baseline and periodic monitoring should include: blood pressure; fasting lipid profile (HDL/LDL/triglycerides); liver function tests (ALT, AST, bilirubin); renal function (creatinine, eGFR); complete blood count (hematocrit/hemoglobin); serum total testosterone, LH, FSH; and PSA in older men or when indicated.
- Evaluate cardiovascular risk factors (history, ECG as clinically indicated) and counsel regarding smoking, diet, and lipid management.
- Women and adolescents: strongly advise against use due to risk of irreversible virilization and effects on growth and development.
- Clinical management of exposure or adverse effects: treatment focuses on stopping exposure, addressing acute complications (e.g., hypertensive crisis, thrombotic events, hepatic injury), and specialist referral (endocrinology, cardiology, hepatology, psychiatry) as indicated.
- Counseling: clinicians should counsel patients that doses reported in non‑medical contexts often far exceed doses of approved anabolic agents, and that such use substantially increases risk of severe adverse events. Any consideration of anabolic steroid therapy should be under specialist supervision with appropriate indication and monitoring.
(Deliberately omitted: step‑by‑step administration instructions, recommended injection regimens, or cycle prescriptions, because trenbolone enanthate is not an approved human medication and providing how-to dosing or administration instructions for off-label/illicit use would be unsafe and potentially unlawful.)
4. Side effects — common and rare/serious adverse effects
Trenbolone is a potent AAS with a broad adverse effect profile. Severity depends on dose, duration, individual susceptibility, co-administered drugs, and comorbidities.
Common / frequently reported effects
- Endocrine:
- Suppression of endogenous testosterone, decreased libido (may be transient or persistent), testicular atrophy, impaired spermatogenesis and potential infertility.
- In women: virilization (deepening of voice, clitoromegaly, hirsutism, menstrual disruption); some changes may be irreversible.
- Dermatologic:
- Acne, oily skin, exacerbation of pre‑existing acne.
- Male-pattern hair loss in genetically susceptible individuals.
- Psychiatric/behavioral:
- Mood changes, increased irritability or aggression, agitation, decreased impulse control, anxiety, depressive symptoms on withdrawal.
- Cardiovascular/metabolic:
- Adverse lipid changes (decreased HDL, increased LDL), which raise ASCVD risk.
- Elevated blood pressure.
- Hematologic:
- Increased hematocrit/hemoglobin (polycythemia), which increases risk for thrombotic events.
- Other:
- Insomnia, night sweats, decreased exercise tolerance in some users.
Rare but serious effects
- Cardiovascular acute events: myocardial infarction, stroke, cardiomyopathy (dilated or hypertrophic), sudden cardiac death—particularly in those with preexisting disease or high‑risk behaviors.
- Hepatic injury: although injectable non‑17α-alkylated AAS are generally less hepatotoxic than 17α-alkylated oral steroids, severe liver dysfunction (cholestasis, hepatic enzyme elevations, rare hepatic failure) has been reported with AAS use.
- Renal injury: focal segmental glomerulosclerosis (FSGS) and other kidney injury syndromes have been associated with long‑term, high‑dose AAS use, possibly exacerbated by increased muscle breakdown and elevated creatine kinase in exertional states.
- Progestogenic effects: despite lack of aromatization, progestogenic activity can contribute to gynecomastia or other endocrine disturbances.
- Infections and complications from nonsterile use: if injected in nonsterile conditions or with shared equipment, risks include localized abscesses, cellulitis, sepsis, and blood-borne infections (HIV, hepatitis B/C).
Signs that require urgent medical attention
- Chest pain, shortness of breath, sudden weakness/numbness, slurred speech (possible myocardial infarction or stroke).
- Jaundice, severe abdominal pain (possible severe hepatic injury).
- Sudden vision changes, severe headache, seizures.
- Very high blood pressure readings, syncope.
- Any signs of a spreading skin infection, high fevers, or signs of sepsis.
Drug interactions and contraindications
- Contraindicated in pregnancy and breastfeeding (risk of virilization of the fetus/infant).
- Contraindicated or relatively contraindicated in people with prostate cancer, severe cardiovascular disease, severe liver disease, and uncontrolled hypertension.
- May interact with anticoagulants (altering coagulation), antidiabetic agents (altering glucose metabolism), corticosteroids (additive catabolic/anabolic interactions), and other drugs affecting lipids or blood pressure.
Long-term considerations
- Persistent hypogonadism after discontinuation is reported and can require endocrine assessment and treatment.
- Long-term AAS use increases lifetime cardiovascular risk and may accelerate atherosclerosis.
- Psychological dependence and substance use disorder are possible.
5. Storage — how to store trenbolone-enanthate formulations
- Store in the original, labeled container away from children and pets.
- Typical recommended conditions for oil-based injectable steroids: controlled room temperature, generally between 15–25 °C (59–77 °F), unless the manufacturer label specifies otherwise. Avoid extreme heat and do not freeze.
- Protect from light and excessive moisture; keep the cap/container closed when not in use.
- Inspect prior to use: do not use if the solution is discolored, cloudy (unless the product is specifically intended to be a sterile oil suspension that is normally clear), or contains particulate matter, or if the vial/packaging is damaged.
- Follow the product’s expiration date; do not use expired material.
- Disposal: follow local regulations for pharmaceutical and sharps disposal. Do not dispose of injectable materials or needles in household waste. Use designated sharps containers and community take‑back programs where available.
Final clinical advice
- Because trenbolone enanthate is not an approved human medicine in many jurisdictions and has a substantial adverse-effect profile, it should not be used outside of regulated veterinary contexts or without appropriate prescription and specialist oversight where legally permitted.
- If you, a patient, or someone you know is using trenbolone-enanthate or other anabolic steroids, encourage disclosure to healthcare providers so appropriate monitoring and management can be provided. For acute problems (cardiac symptoms, jaundice, signs of infection, severe psychiatric symptoms), seek emergency medical care.
- For questions about management of AAS-related health issues, referral to an endocrinologist, cardiologist, hepatologist, or addiction medicine specialist is appropriate.
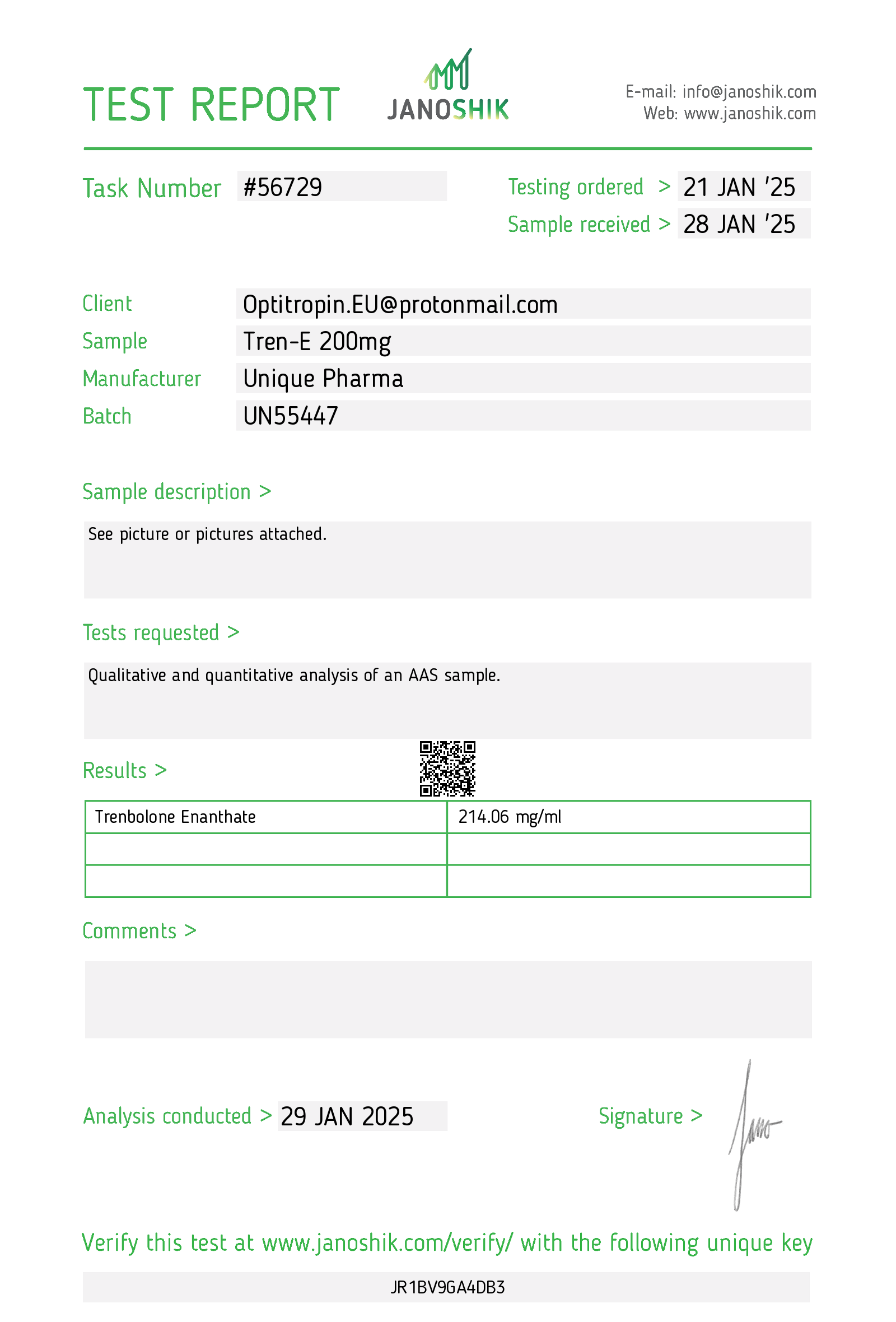
1. Description (Clinical summary)
Tren E 200 mg/mL — trenbolone enanthate — is an oil-based injectable formulation of trenbolone, a synthetic anabolic-androgenic steroid (AAS). Trenbolone is a highly potent androgen with strong anabolic effects and significant androgenic and progestogenic activity. The enanthate ester prolongs release from the injection site, producing a multi-day duration of action compared with shorter esters.
Trenbolone has established use in veterinary medicine (growth promotion in cattle) and is not approved as a therapeutic for humans in many jurisdictions. Use in people is off-label, unapproved, and carries substantial medical and legal risks. The material below is educational and does not constitute a recommendation to use this compound.
2. How does trenbolone-enanthate work? (Mechanism of action)
- Androgen receptor agonist: Trenbolone binds the androgen receptor with high affinity, activating transcriptional programs that increase protein synthesis, muscle fiber accretion, and nitrogen retention.
- Strong anabolic and androgenic effects: Compared with testosterone, trenbolone has a greater anabolic-to-androgenic potency ratio in many experimental settings.
- Non-aromatizing but progestogenic: Trenbolone does not convert to estrogen via aromatase, so it does not raise estradiol directly. However, it has intrinsic progestogenic activity, which can interact with estrogenic pathways and in some people contribute to gynecomastia-like symptoms.
- Catabolic suppression: Trenbolone diminishes glucocorticoid receptor–mediated catabolism, favoring net anabolism.
- Erythropoiesis and metabolism: It stimulates red blood cell production (increasing hematocrit/hemoglobin) and alters lipid and glucose metabolism (typically lowering HDL, raising LDL, and sometimes impairing insulin sensitivity).
- Pharmacokinetics with enanthate ester: The enanthate ester increases lipophilicity and slows release; effective plasma levels are often sustained for about 7–14 days per injection, with steady state requiring several weeks of repeated dosing.
3. Dosage (medical and usage considerations)
Important: there is no approved therapeutic dosing regimen for trenbolone in humans in most countries. The following are reported non-medical/anecdotal patterns from bodybuilding literature (not medical advice). Use is associated with significant risk, including permanent adverse effects.
- Medical: No approved human medical dose. Trenbolone is primarily a veterinary product.
- Reported non-medical ranges (anecdotal):
- Lower-range: ~100–200 mg per week (sometimes split into two injections).
- Commonly reported: ~200–400 mg per week.
- Higher-risk ranges: >400 mg per week (markedly increases adverse-event risk).
- Frequency and cycle length:
- Typical injection frequency with enanthate ester: every 7 days or every 3–4 days (to reduce peak–trough fluctuations).
- Frequently reported cycle durations: 6–12 weeks. Prolonged use beyond these durations increases risks of cumulative adverse effects.
- Co-therapy and endocrine considerations:
- Trenbolone strongly suppresses the hypothalamic–pituitary–gonadal (HPG) axis. Users commonly experience marked suppression of endogenous testosterone production; exogenous testosterone is often used concurrently by non-medical users to maintain androgenicity and reduce hypogonadal symptoms.
- Post-cycle recovery: cessation typically requires medical oversight and monitoring; “post-cycle therapy” interventions are commonly discussed in non-medical circles but should be managed by a clinician.
- Monitoring and precautions:
- Baseline and periodic labs are essential if use occurs: CBC (hematocrit/hemoglobin), fasting lipid profile, liver enzymes (AST/ALT), renal function (creatinine/BUN), fasting glucose/HbA1c, total testosterone, LH/FSH, and PSA in older men. Blood pressure and ECG evaluation are also recommended if cardiovascular risk exists.
- Contraindications include: established cardiovascular disease, severe hypertension, active prostate or breast cancer, pregnancy, breastfeeding, adolescence (growth not complete), and severe hepatic or renal impairment.
Because of legal, ethical, and medical risks, any consideration of exposure should be discussed with a licensed clinician and monitored with appropriate laboratory and clinical follow-up.
4. Side effects (common and rare / serious adverse effects)
Trenbolone carries a high side-effect burden. Effects vary with dose, duration, genetics, sex, and co-administered compounds.
Common/expected adverse effects
- Endocrine:
- Marked suppression of endogenous testosterone production, testicular atrophy, reduced sperm count and fertility.
- Decreased libido (may paradoxically increase in some short-term users).
- Androgenic/dermatologic:
- Acne, oily skin, accelerated male pattern hair loss in genetically susceptible individuals, increased body/facial hair.
- Virilization in females (voice deepening, clitoral enlargement, menstrual irregularities) — often irreversible.
- Cardiovascular / metabolic:
- Adverse lipid changes: reduced HDL, increased LDL; increased atherosclerotic risk over time.
- Elevated blood pressure.
- Hematologic:
- Polycythemia (increased hematocrit/hemoglobin) — raises risk of thrombosis.
- Neuropsychiatric:
- Mood changes, increased irritability or aggression, anxiety, insomnia, occasional mania or severe mood disturbances.
- Injection-related:
- Pain, swelling, or infection at injection sites (abscess, cellulitis) with improper technique.
Less common but serious risks
- Major cardiovascular events: myocardial infarction, stroke; particularly in those with underlying risk factors or prolonged high-dose use.
- Thromboembolic events (deep vein thrombosis, pulmonary embolism), sometimes associated with polycythemia.
- Hepatic injury: trenbolone is not a classic 17α-alkylated oral hepatotoxin but can still affect liver enzyme levels and contribute to hepatic dysfunction in susceptible individuals or with concomitant hepatotoxic substances (alcohol, other hepatotoxins).
- Renal stress/failure: reported rarely, sometimes associated with rhabdomyolysis or prolonged high-dose use.
- Severe psychiatric effects: psychosis, severe depression, violence, or suicidal ideation in rare cases.
- Gynecomastia: secondary to progestogenic effects and altered estrogen signaling in some users.
- Permanent virilization in females if exposure occurs.
Red flags requiring urgent medical attention
- Chest pain, sudden shortness of breath, sudden severe headache, focal neurological deficits (possible stroke), acute severe abdominal pain, jaundice, markedly dark urine, signs of deep vein thrombosis (limb swelling/redness), seizures, or severe psychiatric changes (suicidal or homicidal ideation).
5. Storage (how to store it)
- Keep in original, labeled container and store at controlled room temperature, typically 20–25 °C (68–77 °F). Avoid extreme heat or cold; do not freeze.
- Protect from prolonged exposure to direct sunlight.
- Keep out of reach of children and pets; store securely and in compliance with local regulations for controlled substances.
- Inspect vials before use: do not use if solution is discolored, cloudy (unless product is intended to be clear), or contains particulate matter; do not use if the seal or vial integrity is compromised.
- Single-use vs. multi-dose: follow the manufacturer’s labeling for multi-dose vials and recommended timeframes for needle entry; use aseptic technique and discard if contamination is suspected.
- Disposal: dispose of sharps (needles, syringes) and unused medication per local regulations (e.g., sharps containers, authorized take-back programs); do not flush down the toilet.
Additional important considerations
- Legal status: trenbolone is a controlled substance in many countries and is often restricted to veterinary use. Possession, distribution, or use without appropriate authorization may be illegal.
- If you or someone has used trenbolone and experiences concerning symptoms, seek medical evaluation promptly. If use is ongoing, discuss discontinuation and supervised medical follow-up with a licensed healthcare provider.
- Safer alternatives: For clinically appropriate anabolic needs (e.g., severe wasting), prescriptions for approved therapies (testosterone replacement, medically supervised anabolic therapy) exist and should be discussed with a physician.
If you’d like, I can summarize typical laboratory monitoring schedules, list baseline tests to request from a clinician, or provide a checklist of warning signs to watch for during use.