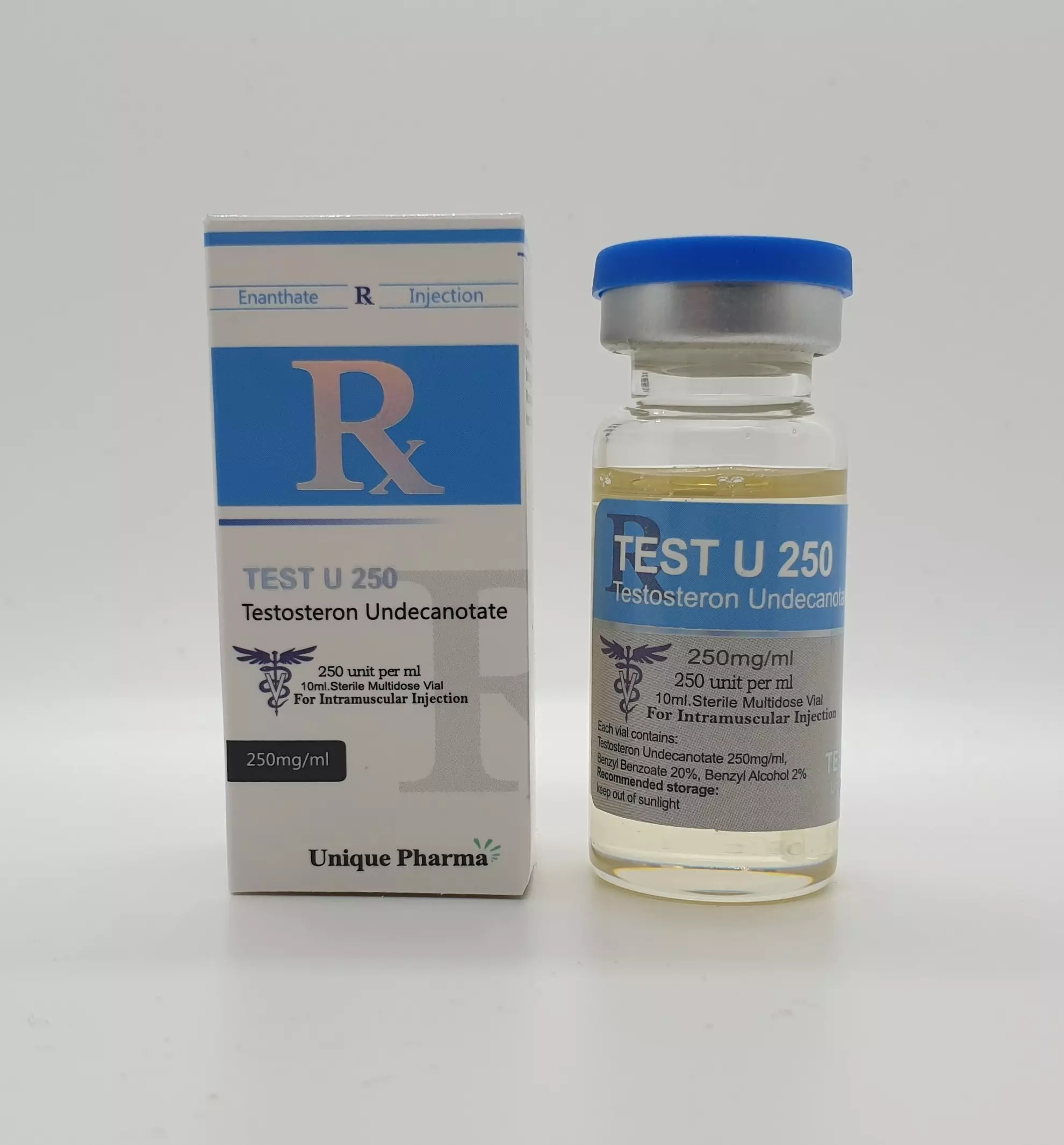

Testosterone Undecanoate 250 mg/ml (UP)
€ 45,00
Op voorraadBuy premium Testosterone Undecanoate from Unique Pharma. Lab-tested, fast shipping, competitive prices.
Acne
Yes
Halfwaardetijd
8 Days
Dosering
250-500mg Weekly
Detectietijd
90 Days
Aromatisering
Yes
Water Retentie
Yes
Hepatotoxiciteit
Yes
HBR
Yes
Product Informatie
Over Testosterone Undecanoate 250 mg/ml (UP)
1. Description (Clinical summary)
Testosterone undecanoate (TU) is a long‑acting injectable ester of testosterone used primarily for testosterone replacement therapy (TRT) in adult males with confirmed hypogonadism (primary or hypogonadotropic). It is a highly lipophilic prodrug formulated in an oil vehicle for deep intramuscular (IM) injection; after depot release it is hydrolyzed to active testosterone. TU restores and maintains physiologic testosterone concentrations, improving symptoms such as low libido, fatigue, loss of muscle mass, decreased bone mineral density and mood disturbances associated with testosterone deficiency.
Licensed long‑acting TU products are dosed at relatively large single volumes (e.g., 1000 mg per injection delivered as 4 mL of a 250 mg/mL formulation in commonly used preparations). Use of TU should be under the supervision of a healthcare professional with appropriate baseline evaluation and ongoing monitoring.
Indications (typical)
- Adult men with symptomatic hypogonadism and documented low serum testosterone.
- Uses and regimens in women or children are generally contraindicated because of virilizing effects except in very specific, specialist‑supervised circumstances.
2. How does testosterone‑undecanoate work? (Mechanism of action)
- TU is an esterified form of testosterone (testosterone attached to an undecanoate ester). After IM depot administration, tissue and plasma esterases cleave the undecanoate ester to release free testosterone.
- Testosterone binds androgen receptors (AR) in target tissues (muscle, bone, brain, prostate, hair follicles, etc.). The ligand‑receptor complex translocates to the nucleus and modulates transcription of androgen‑responsive genes, producing androgenic and anabolic effects (sexual function, erythropoiesis, protein synthesis, bone metabolism).
- Testosterone can be locally converted to:
- Dihydrotestosterone (DHT) via 5α‑reductase (potent androgen with actions in prostate, skin, hair).
- Estradiol via aromatase (important for bone health, fat distribution and feedback on hypothalamic‑pituitary axis).
- Exogenous testosterone suppresses the hypothalamic‑pituitary‑gonadal axis, reducing luteinizing hormone (LH) and follicle‑stimulating hormone (FSH), which decreases intratesticular testosterone and spermatogenesis (fertility suppression).
3. Dosage (Medical and usage guidelines)
Important general points
- Use only under physician supervision with confirmed low testosterone and appropriate baseline evaluation. Individualize dose and dosing interval according to product labeling, clinical response and serum testosterone measurements.
- Do not substitute dosing regimens between different testosterone formulations without medical guidance — TU has a long depot action and specific licensed schedules.
Typical licensed regimen for long‑acting injectable TU (example for 250 mg/mL concentration)
- Commonly used preparation: 250 mg/mL oil solution packaged as 1000 mg in 4 mL (4 mL × 250 mg/mL = 1000 mg).
- Typical schedule (manufacturer guidance for many TU products, subject to product label):
- Initial injection: 1000 mg (4 mL) IM.
- Second injection: 1000 mg IM at 6 weeks.
- Maintenance injections: 1000 mg IM every 10–14 weeks thereafter (interval individualized to maintain target serum testosterone and symptom control).
- Administration: deep intragluteal IM injection (see section on administration and precautions). Some regimes require observation for immediate adverse events (see safety).
Monitoring and dose adjustment
- Measure serum total testosterone (ideally trough concentration, i.e., immediately before the next scheduled injection) and clinical response to guide interval adjustments.
- If trough testosterone consistently below target, shortening the interval within licensed limits or re-evaluating injection technique/adherence may be required. Any change should be directed by a prescriber.
- Avoid using short‑acting testosterone dosing approaches based on TU without clinician oversight.
Special populations and cautions
- Women: TU is contraindicated for routine use because of virilization; only used in specialist settings when benefits outweigh risks (very rare).
- Fertility: testosterone therapy suppresses spermatogenesis. Men desiring fertility should be counseled and offered alternative approaches (e.g., gonadotrophin therapy).
- Elderly: start with caution; monitor cardiovascular, prostate and hematologic status closely.
- Pediatrics: not indicated except in specific, specialist‑managed conditions (e.g., delayed puberty), using lower doses and specialist guidance.
Off‑label and nonmedical dosing
- Medical guidance should explicitly discourage nonmedical or supratherapeutic use (e.g., for performance enhancement). Such use increases risk of serious adverse outcomes (cardiovascular events, polycythemia, hepatic and endocrine disturbances) and may be illegal.
4. Side effects
Common and expected adverse effects
- Injection site reactions: pain, discomfort, erythema, rarely local nodules.
- Androgenic effects: acne, oily skin, increased facial/body hair, male pattern hair changes.
- Increased libido, mood changes (improved or sometimes irritability/aggression).
- Fluid retention and peripheral edema (mild).
- Increased hemoglobin and hematocrit (erythrocytosis/polycythemia).
- Testicular atrophy and decreased sperm production (reversible in many cases after discontinuation but may be prolonged).
- Gynecomastia (due to aromatization to estradiol) in some patients.
Less common but serious adverse effects (require prompt medical attention)
- Cardiovascular: increased risk of venous thromboembolism (deep vein thrombosis, pulmonary embolism) and possible association with adverse cardiovascular events (myocardial infarction, stroke) in certain populations — monitor risk factors and intervene as indicated.
- Hypertension: new or worsened high blood pressure.
- Hepatic dysfunction: injectable TU is less hepatotoxic than some oral formulations, but monitor liver tests if clinically indicated.
- Prostate effects: stimulation of benign prostatic hyperplasia (BPH) symptoms and potential stimulation of occult prostate cancer — baseline PSA and digital rectal exam (DRE) recommended in appropriate age groups; avoid use in known prostate or male breast cancer.
- Pulmonary oil microembolism (POME) and hypersensitivity: rare immediate reactions (cough, dyspnea, throat tightness, chest pain, dizziness) reported with oil‑based IM testosterone injections; can be abrupt — observe patients post‑injection if required by local product safety guidance.
- Priapism (prolonged painful erections).
- Severe allergic reactions (rare): angioedema, anaphylaxis.
Monitoring to reduce risk
- Baseline: serum total testosterone, hematocrit/hemoglobin, PSA (men >40 or per local guidance), liver function tests, lipid profile, blood pressure, clinical assessment for cardiovascular risk, and digital rectal exam as indicated.
- After initiation: check hematocrit at 3–6 months and then periodically (elevated Hct >54% often prompts dose reduction/temporary cessation or phlebotomy). Check testosterone levels 2–12 weeks after initial dosing and prior to subsequent injections to guide timing; PSA and DRE at 3–6 months then yearly as appropriate.
- Advise patients to report signs of blood clots, chest pain, shortness of breath, significant mood changes, symptoms of prostate enlargement, jaundice, or priapism.
Contraindications (important)
- Known or suspected prostate cancer or male breast cancer.
- Hypersensitivity to testosterone or formulation excipients.
- Pregnancy and lactation (women exposed to androgenic compounds risk fetal virilization).
Drug interactions
- TU can alter the effects of anticoagulants (monitor INR in warfarin users).
- Concomitant corticosteroids may increase edema risk.
- Drugs that influence hepatic enzymes may alter testosterone metabolism (monitor clinical effect).
Emergency advice
- For signs of severe allergic reaction (difficulty breathing, swelling of face/lips/throat), seek immediate emergency care.
- For symptoms suggestive of thrombosis (leg swelling/pain, chest pain, sudden breathlessness) seek urgent medical assessment.
5. Storage (how to store it)
- Store TU injection at room temperature as specified by the product labeling, typically 20–25 °C (68–77 °F). Short excursions within manufacturer guidance are acceptable; avoid extremes of temperature.
- Protect from light and keep the vial in its original container until use.
- Do not freeze. Do not use if the solution is discolored, cloudy (unless the product is intended to be an oil‑based clear solution) or contains particulate matter.
- Keep out of reach of children and pets.
- Follow local regulations for disposal of unused medication and sharps (needles, syringes). Do not reuse needles or syringes.
- Check the expiry date on the packaging; do not use past expiration.
Final clinical notes
- Testosterone undecanoate is an effective long‑acting agent for TRT when used according to approved indications and dosing schedules. Because of its potent systemic effects (androgenic, erythropoietic, cardiovascular and prostate‑related), initiation and ongoing therapy require appropriate baseline assessment and periodic monitoring by a qualified healthcare professional. Any changes in symptoms or laboratory values should prompt clinical reassessment.
Dosering
Aanbevolen
250-500mg Weekly
Halfwaardetijd
8 Days
Voordelen
- Laboratorium getest op zuiverheid
- Farmaceutische kwaliteit gegarandeerd
- Discrete en veilige verzending
- Uitstekende klantenservice
- Snelle levering in heel Europa
Gratis verzending
Gratis verzending bij bestellingen boven de €200.
Leveringsgarantie
Gratis herverzending als uw bestelling niet aankomt.
Snelle levering
Verzending binnen 24u. Levering 48-72u in NL & BE.
Bitcoin Betaling
Betaal veilig en blijf volledig anoniem met Bitcoin.
Veilig afrekenen
Beveiligde SSL-verbinding voor alle transacties.
Authentiek
Originaliteitscontrole voor al onze producten.