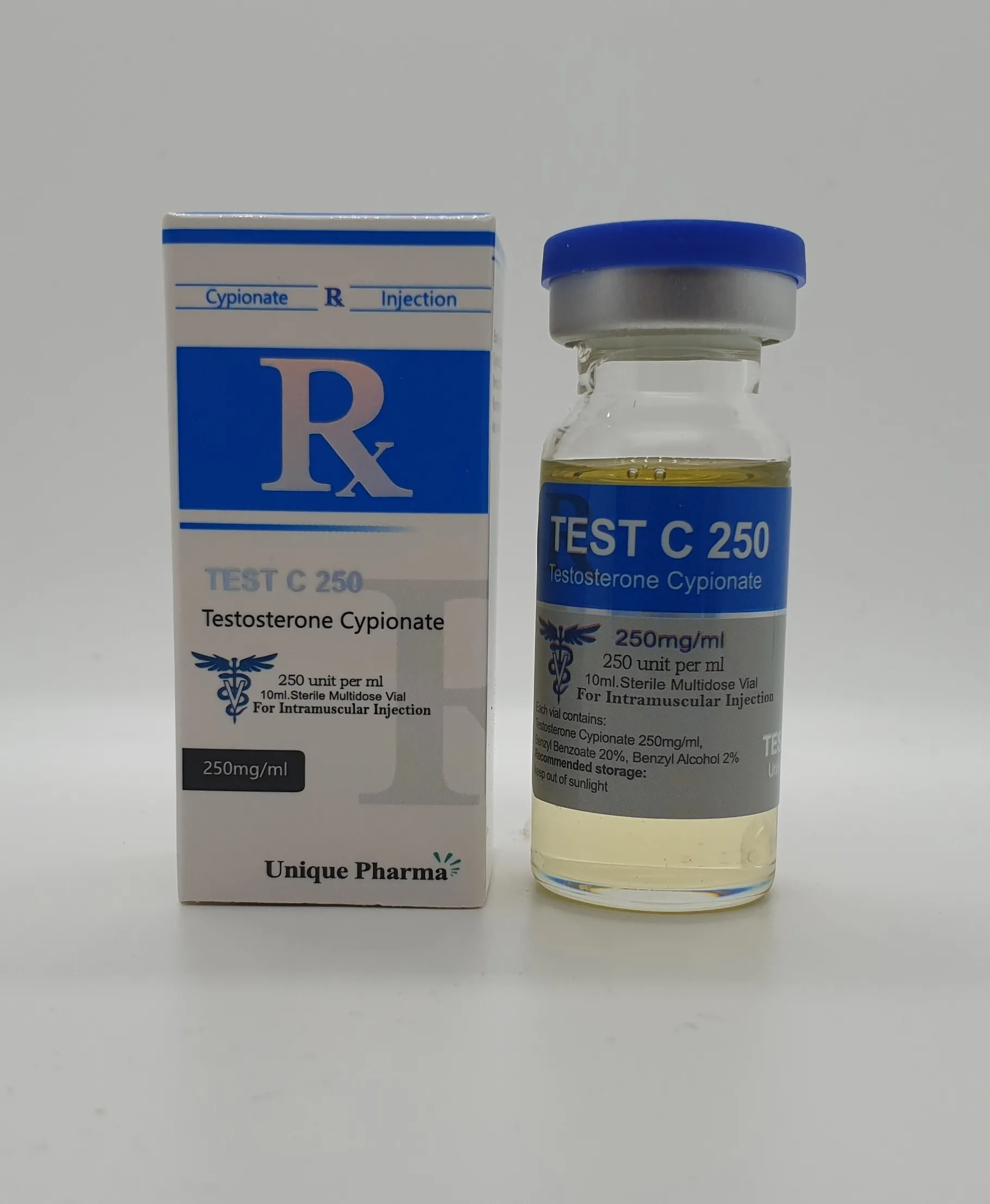
UNIQUE PHARMA QUALITY: Premium pharmaceutical grade Testosterone Cypionate (Test Cyp) manufactured under strict GMP conditions with 99.8% purity verification.
Test Cyp from Unique Pharma represents our commitment to delivering exceptional quality performance enhancement compounds. Each batch undergoes rigorous testing to ensure consistent potency and purity standards.
Key Characteristics of Test Cyp
This injectable compound is administered via intramuscular injection and remains active in your system for approximately 8 Days. Notable features include:
- Pharmaceutical grade manufacturing
- Batch-tested for purity and potency
- Consistent dosing per unit
- Optimal bioavailability
Primary Benefits:
- Enhanced performance and recovery
- Quality-assured formulation
- Reliable and consistent results
- Professional-grade compound
Mechanism of Action
Testosterone Cypionate works by interacting with androgen receptors in muscle tissue, promoting protein synthesis and nitrogen retention. This creates an optimal environment for muscle development and recovery. The compound's unique molecular structure provides specific benefits that distinguish it from other options in its class.
Usage Guidelines
Unique Pharma Test Cyp is suitable for experienced users who understand proper cycling protocols. Always consult with a healthcare professional before beginning any supplementation regimen. Proper post-cycle therapy should be considered based on individual needs and cycle duration.
Recommended Applications
This compound is commonly incorporated into both bulking and cutting protocols depending on the user's specific goals. Its versatility makes it a popular choice among athletes and bodybuilders seeking reliable results.
Potential Considerations
As with any performance compound, users should be aware of potential effects and monitor their response accordingly. Regular health monitoring is recommended during use. Individual responses may vary based on genetics, diet, training, and other factors.
Quality Assurance
Every Unique Pharma product undergoes comprehensive quality control including:
- Raw material verification
- In-process testing
- Final product analysis
- Stability testing
Warning: Keep out of reach of children. For adults only. Not intended for use by individuals under 18 years of age.
Related products
Other Unique Pharma products
1. Description: Clinical summary
Test C 250 mg/mL (active compound: testosterone cypionate) is an oil‑based intramuscular (IM) injectable formulation of testosterone ester. It is an androgen indicated primarily for replacement therapy in adult males with clinical evidence of testosterone deficiency (hypogonadism) confirmed by low serum testosterone and compatible signs/symptoms (reduced libido, fatigue, loss of muscle mass, osteoporosis, etc.). Testosterone cypionate is a long‑acting ester of testosterone; after IM injection the ester is slowly hydrolyzed to active testosterone.
Use should be by, or under the supervision of, a qualified healthcare professional. Testosterone preparations are controlled substances in many jurisdictions and have important contraindications, monitoring requirements, and potential serious adverse effects.
2. How does testosterone‑cypionate work?: Mechanism of action
- Testosterone cypionate is a prodrug (esterified testosterone). Following intramuscular administration the cypionate ester is hydrolyzed, releasing native testosterone into the circulation.
- Testosterone binds to androgen receptors (nuclear receptors) in target tissues. The receptor‑ligand complex modulates gene transcription, leading to androgenic and anabolic effects:
- Androgenic effects: development/maintenance of male secondary sexual characteristics (libido, libido, body hair, voice, etc.).
- Anabolic effects: increased protein synthesis, positive nitrogen balance, increased muscle mass and strength, and effects on bone mineralization.
- Exogenous testosterone suppresses the hypothalamic–pituitary–testicular (HPT) axis via negative feedback, lowering gonadotropin (LH/FSH) secretion and often decreasing intratesticular testosterone and spermatogenesis.
3. Dosage: Medical and varying usage guidelines
General principles and cautions
- Dosage must be individualized based on the indication, baseline testosterone levels, clinical response, age, comorbidities, and monitoring results.
- Only use when hypogonadism has been confirmed. Not indicated for age‑related declines without clear clinical and biochemical evidence.
- Avoid use in patients with prostate or breast cancer, or in pregnant women.
Typical medically recommended adult male dosing (replacement therapy)
- Concentration: commonly available as 250 mg/mL.
- Common regimens:
- 50–100 mg IM every week, or
- 100–200 mg IM every 1–2 weeks.
- Many clinicians prescribe 100 mg weekly (or 200 mg every 2 weeks) to provide more stable serum levels and reduce symptom fluctuation; smaller, more frequent dosing tends to produce steadier levels.
- Titration: start at a standard replacement dose, then adjust to achieve mid‑normal serum total testosterone and symptomatic improvement while minimizing adverse effects.
- Measurement: obtain baseline testosterone, then repeat serum total testosterone at a consistent timing relative to injection (for example just before the next dose [trough] or a specified day post‑injection) to guide dose adjustments. Aim for physiologic mid‑normal ranges (typical target ~300–800 ng/dL; local reference ranges vary).
Special populations and other considerations
- Older adults: start low and monitor carefully for cardiovascular, hematologic, and prostate effects.
- Women: injectable testosterone formulations like cypionate are generally not routinely recommended for women; if androgen therapy is considered (e.g., for hypoactive sexual desire disorder), use specialist guidance and typically much lower doses or alternative formulations.
- Adolescents/pediatrics: only under specialist care for specific indications (e.g., delayed puberty), with specific age‑appropriate dosing.
- Fertility: exogenous testosterone suppresses spermatogenesis—do not use as contraception and avoid if fertility is desired; discuss alternatives (e.g., gonadotropin therapy).
Non‑medical (performance‑enhancing) use
- Many non‑medical regimens for bodybuilding or athletic enhancement use much higher and more frequent doses than therapeutic ranges; these carry substantially increased risks (cardiovascular, hepatic, hematologic, psychiatric, endocrine). Such use is unsafe and not recommended.
Administration notes
- Route: deep intramuscular injection (typically gluteus maximus or lateral thigh). Use aseptic technique.
- Needle size commonly used in adults: 21–23 gauge; 1–1.5 inch (25–38 mm) length for gluteal IM injection (adjust based on body habitus). Healthcare providers should provide injection training if self‑administration is contemplated.
- Rotate injection sites. Inspect solution for particulate matter or discoloration; do not use if contaminated.
Monitoring recommendations (typical)
- Baseline: total testosterone, CBC (hematocrit/hemoglobin), PSA (men >40 or with risk), liver function tests, fasting lipids, glucose/HbA1c, and assessment for cardiovascular risk and prostate symptoms.
- After initiation: testosterone level and symptom assessment at 3–4 months, CBC at 3 months then every 6–12 months, PSA and digital rectal exam per prostate cancer screening guidelines or earlier if indicated.
- Monitor blood pressure, weight, mood/behavior changes, and signs of polycythemia (hematocrit >54% is concerning).
- If hematocrit rises significantly, dosing interval reduction, dose reduction, or therapeutic phlebotomy may be needed.
When to seek medical attention
- Sudden chest pain, severe shortness of breath, signs of thrombosis, jaundice, severe allergy, or other acute severe symptoms require immediate medical attention.
4. Side effects: Common and rare adverse effects
Common and expected effects
- Injection‑site pain or irritation.
- Acne, oily skin, increased facial/body hair.
- Increased appetite and weight gain.
- Fluid retention and peripheral edema.
- Increased hematocrit/polycythemia (risk of thrombosis if severe).
- Suppression of spermatogenesis, reduced testicular size.
- Altered lipid profile (often decreased HDL, variable LDL/triglycerides).
- Mood lability, irritability, aggression in some patients.
Less common but clinically important
- Gynecomastia (aromatization to estrogen).
- Erectile dysfunction may persist in some despite therapy.
- Worsening of sleep apnea.
- Hypertension exacerbation.
- Elevated liver enzymes (less common with IM esters than with oral 17‑alkylated androgens).
Rare but severe
- Venous thromboembolism (deep vein thrombosis, pulmonary embolism).
- Myocardial infarction, stroke — there is ongoing debate and evidence that exogenous testosterone may increase cardiovascular risk in some patients, especially at higher doses or with preexisting disease.
- Severe allergic reactions (anaphylaxis) to formulation components.
- Cholestatic jaundice and hepatic dysfunction (rare with injectable testosterone but reported).
- Psychiatric effects: severe mood disorders, mania, or aggression in susceptible individuals.
Drug interactions and special warnings
- May increase effects of oral anticoagulants (warfarin) and require INR monitoring/adjustment.
- Concomitant use with systemic corticosteroids may increase risk of fluid retention and edema.
- Use with caution in patients with severe cardiac, hepatic, or renal disease.
- Contraindicated in men with known or suspected prostate or breast carcinoma.
5. Storage: HOW to store it
- Store at controlled room temperature (typically 20–25 °C / 68–77 °F). Check the product label for the exact recommended temperature range.
- Protect from light. Do not freeze.
- Keep in original container until use.
- Do not use if the solution is discolored, cloudy (if it should be clear), or contains particulate matter.
- Keep out of reach of children and pets. Dispose of needles, syringes, and unused product safely per local regulations for sharps and controlled substances.
- Observe any expiration date printed on the vial; do not use after expiration.
Key reminders
- Testosterone cypionate is prescription‑only and should be used only under medical supervision with appropriate baseline assessment and periodic monitoring.
- Discuss fertility desires, cardiovascular risk, prostate cancer risk, and potential adverse effects with a healthcare provider before starting therapy.
- Report any concerning symptoms (chest pain, sudden shortness of breath, signs of thromboembolism, jaundice, severe mood changes, or marked polycythemia) promptly.
If you want, I can summarize typical monitoring timelines, list common contraindications in checklist form, or provide a sample baseline lab panel to discuss with your clinician.
1. Description
Test C 250 mg/mL (active compound: testosterone cypionate) is an injectable androgen preparation consisting of testosterone esterified with cypionic acid. It is formulated as an oil-based intramuscular (IM) depot that releases testosterone slowly after IM administration. Clinically it is used primarily for replacement therapy in males with confirmed hypogonadism (primary or secondary) and for other medically indicated situations where androgen therapy is appropriate (e.g., delayed puberty in males under specialist supervision, certain gender-affirming regimens under the care of a knowledgeable clinician). Testosterone cypionate is a controlled substance that should be used only under appropriate medical supervision.
Key formulation points:
- Concentration commonly 250 mg of testosterone cypionate per mL.
- Oil-based IM injection; depot effect prolongs systemic exposure compared with unesterified testosterone.
2. How does testosterone-cypionate work?
- Testosterone cypionate is a prodrug: the cypionate ester confers lipophilicity allowing slow release from the injection site. Esterases in blood and tissues cleave the ester to release active testosterone.
- Active testosterone binds to androgen receptors in target tissues. The receptor–ligand complex modulates transcription of androgen-responsive genes, producing anabolic (e.g., increased protein synthesis, lean mass) and androgenic (e.g., development/maintenance of male secondary sexual characteristics) effects.
- Testosterone can be converted peripherally:
- To dihydrotestosterone (DHT) via 5α-reductase (potent androgenic actions, especially in skin and prostate).
- To estradiol via aromatase (mediates some effects on bone, fat distribution, and can contribute to gynecomastia).
- Net physiologic effects include masculinization, increased libido, maintenance of muscle mass and bone density, and suppression of the hypothalamic–pituitary–gonadal axis (decreased LH and FSH with consequent decreased intratesticular testosterone and potential spermatogenesis suppression).
3. Dosage
All dosing must be individualized and supervised by a clinician. Baseline evaluation (see Section 4 and monitoring) is required before initiating treatment.
Typical therapeutic dosing (adult males, hypogonadism):
- Common prescribing range: 50 to 400 mg IM every 2 to 4 weeks (historically in product labeling).
- Practical regimens commonly used in contemporary practice:
- 100 mg IM every 7 days (weekly), or
- 100–200 mg IM every 1–2 weeks (biweekly dosing such as 200 mg every 2 weeks).
- Some clinicians prefer smaller, more frequent dosing (e.g., weekly) to reduce peak–trough fluctuations and symptoms of mood/energy variability.
Transgender men (female-to-male gender-affirming care):
- Typical cross-sex hormone regimens use similar dosing ranges (e.g., 50–100 mg IM weekly or 100–200 mg IM every 2 weeks), but regimens are individualized and coordinated with a gender-health provider.
Delayed puberty in adolescent males:
- Doses are lower and titrated by pediatric/endocrinology specialists (e.g., small incremental IM doses to induce puberty and avoid premature epiphyseal closure). Pediatric dosing must be managed by specialists.
Special notes:
- For fertility preservation in men desiring future spermatogenesis, exogenous testosterone alone can suppress spermatogenesis — alternative approaches (clomiphene, hCG with/without FSH, or specialist strategies) should be discussed with a reproductive endocrinologist/urologist.
- Do not use in women who are or may become pregnant (teratogenic masculinization). Use in cisgender women is generally contraindicated because of virilizing effects; if used for specific medical indications it must be specialist-managed and doses are much lower.
- If switching between testosterone formulations/esters, monitor clinical response and serum testosterone and adjust dose/timing accordingly.
Administration:
- Deep intramuscular injection (usually gluteal or thigh) by a healthcare professional or a trained patient. Do not inject intravenously. Rotate injection sites. Use proper needle length to ensure deep IM delivery.
- If dosing schedule is changed, allow for the depot pharmacokinetics (drug continues to be released over days).
4. Side effects
Common and less serious (may be dose-related):
- Acne, oily skin, increased facial/body hair
- Increased libido and changes in mood or aggression
- Fluid retention and weight gain
- Injection-site pain, bruising
- Increased hematocrit/hemoglobin (erythrocytosis)
- Suppression of testicular size and spermatogenesis (reversible in many cases after cessation, but recovery may take months)
- Changes in lipid profile (reduced HDL, variable LDL)
- Sleep apnea exacerbation or new/worsening snoring
Serious or uncommon (require prompt medical attention):
- Polycythemia (hematocrit elevation can increase risk of thrombosis/stroke/MI)
- Hypertension and exacerbation of heart failure
- Worsening of benign prostatic hyperplasia (BPH) symptoms; theoretical/observed impact on prostate cancer progression—testosterone is contraindicated in men with known prostate or breast cancer
- Hepatic dysfunction (rare for injectable esters; more commonly associated with 17α-alkylated oral androgens)
- Gynecomastia (via aromatization to estradiol)
- Severe dermatologic reactions or allergic reaction (rare)
- Virilization in women and children exposed to testosterone (voice deepening, clitoromegaly, hirsutism)
- Premature epiphyseal closure in adolescents (can stunt final height if used inappropriately)
Monitoring and management recommendations (general):
- Baseline: total testosterone (morning value), LH/FSH, CBC (hematocrit/hemoglobin), PSA (men >40 or per guidelines), fasting lipids, liver function tests, and baseline blood pressure. Screen for sleep apnea and cardiovascular disease risk.
- Recheck testosterone 1–3 months after initiation or dose change to ensure therapeutic range and adjust dosing/timing to minimize symptoms/peaks/troughs. Thereafter every 6–12 months if stable.
- CBC (hematocrit): check at 3 months, 6 months, then every 6–12 months. If hematocrit >54% (or per local guidance), dose reduction, temporary discontinuation, or therapeutic phlebotomy may be indicated.
- PSA and digital rectal exam per age- and risk-based guidelines (baseline and periodic monitoring).
- Lipids and LFTs periodically.
- If new or worsening cardiovascular symptoms, elevated hematocrit, severe mood changes, or signs of prostate disease occur, stop therapy and evaluate.
Drug interactions and cautions:
- May potentiate the effects of oral anticoagulants — monitor INR closely if co-administered with warfarin.
- May cause fluid retention when combined with corticosteroids or agents that promote sodium retention.
- Alterations in blood glucose control have been reported in diabetic patients — monitor glycemic control.
- Use caution in patients with severe cardiac, hepatic, or renal disease.
Contraindications:
- Known hypersensitivity to testosterone or formulation excipients
- Men with known or suspected prostate or breast cancer
- Pregnant women (teratogenic to female fetus) and breastfeeding should avoid exposure
Legal/precautionary note:
- Testosterone preparations, including testosterone cypionate, are controlled substances in many jurisdictions. Use only according to a prescriber’s order and local law.
5. Storage
- Store at controlled room temperature, typically 20–25°C (68–77°F). Some product labeling allows 15–30°C (59–86°F); follow the specific product insert for the exact range.
- Protect from light; keep the vial in its original carton if provided.
- Do not freeze. If frozen, do not use the vial.
- Keep out of reach of children and pets.
- Dispose of needles and syringes in an appropriate sharps container and follow local regulations for disposal of medical waste.
- Do not use if the solution is discolored, contains particulate matter, or if the vial or packaging appears compromised.
This guide is informational and not a substitute for medical advice. Dosing, monitoring, and treatment decisions should be made by a qualified healthcare professional familiar with the patient’s clinical status, comorbidities, and local regulations. If you need references or a clinician-focused monitoring checklist, I can provide one.
1. Description — Clinical summary
Test C 250 mg/mL (active compound: testosterone cypionate) is an oil-based intramuscular depot formulation of testosterone delivered as the cypionate ester. It is used clinically to replace testosterone in males with primary or secondary hypogonadism (congenital or acquired), and in some specialist indications (e.g., part of gender‑affirming hormone therapy for transgender men) under physician supervision.
Testosterone cypionate provides sustained release of testosterone after intramuscular injection with an elimination half‑life that supports injections typically given every 1–4 weeks. It produces systemic androgenic and anabolic effects similar to endogenous testosterone when given at physiologic replacement doses.
2. How does testosterone‑cypionate work? — Mechanism of action
- Testosterone is an androgen that acts via the intracellular androgen receptor (AR). After crossing cell membranes, testosterone (and its intracellular metabolite dihydrotestosterone, DHT, formed by 5α‑reductase) binds AR, leading to receptor activation, nuclear translocation, and modulation of target gene transcription.
- Effects include development and maintenance of male secondary sexual characteristics, stimulation of spermatogenesis (indirectly via gonadotropin regulation), anabolic effects on muscle and bone, and modulation of erythropoiesis (increasing red cell mass).
- Testosterone is aromatized to estradiol in some tissues; this contributes to bone health and can lead to estrogenic effects (e.g., gynecomastia).
- Exogenous testosterone suppresses the hypothalamic‑pituitary‑gonadal axis (↓GnRH, ↓LH, ↓FSH), which may reduce intratesticular testosterone and spermatogenesis and can cause testicular atrophy and infertility with prolonged use.
3. Dosage — Medical and varying usage guidelines
All dosing should be individualized and guided by a qualified clinician. The following are typical medical regimens; they are approximate and not a substitute for specialist prescribing.
General points:
- Formulation: oil-based IM injection, commonly available as 250 mg/mL. Injection volume = dose (mg) ÷ 250 mg/mL.
- Typical therapeutic goal: achieve and maintain serum testosterone in the mid physiologic male reference range (usually measured in the morning).
- Prefer smaller, more frequent dosing (e.g., weekly) to minimize peaks and troughs and associated mood/energy fluctuations.
Recommended typical regimens (adult men):
- Hypogonadism (replacement therapy):
- Common: 50–100 mg IM every week, or
- 100–200 mg IM every 1–2 weeks.
- Historically used: 200 mg every 2 weeks (less favored due to wider concentration fluctuations).
- Transgender men (female‑to‑male), initial/maintenance therapy:
- Frequently used regimens: 50–100 mg IM every week, or 100–200 mg IM every 1–2 weeks; many clinicians use 200 mg every 2 weeks but dose/timing should be individualized and monitored closely.
- Older adults:
- Start at lower end of replacement range; monitor cardiovascular risk, PSA, hematocrit.
- Special populations:
- Pediatrics/delayed puberty: use only under pediatric endocrinologist guidance; much lower, staged dosing is used.
- Women: androgen therapy is generally avoided because of virilization; in very specific, monitored clinical settings doses are much lower and administered by specialists.
Non‑medical/supraphysiologic doses:
- Higher doses used outside medical supervision (e.g., for anabolic effects) are associated with markedly increased risks (cardiovascular events, liver and psychiatric effects, infertility). Such use is not medically recommended.
Monitoring and dose adjustment:
- Check baseline serum testosterone, CBC (hematocrit/hemoglobin), PSA (men >40 or per local practice), liver function tests (LFTs), fasting lipids and glucose as clinically indicated.
- Reassess clinical response and testosterone level about 2–3 months after initiation or after dose change; then periodically (e.g., every 6–12 months) or more often if symptoms or lab changes occur.
- For IM cypionate, measure testosterone at an appropriate time relative to dosing (commonly mid‑interval for weekly dosing or just before the next dose for less frequent schedules) to evaluate troughs/peaks.
Administration technique (brief):
- Route: deep intramuscular injection (gluteal or lateral thigh). Use appropriate needle length and gauge for muscle depth and patient habitus (e.g., 1–1.5 in, 21–23 gauge for gluteal injections in many adults). Follow sterile technique.
- Rotate injection sites. Single‑use syringes and needles; do not share needles. Dispose of sharps safely.
Missed dose:
- If a scheduled dose is missed, administer it as soon as possible and resume dosing schedule; do not double doses without clinician advice. If significant time has passed, consult prescriber for guidance.
4. Side effects — Common and rare adverse effects
Common/expected effects (dose‑dependent):
- Injection site pain, erythema.
- Acne, oily skin.
- Increased libido and sexual function changes.
- Mood changes (irritability, aggression, mood swings).
- Fluid retention and peripheral edema.
- Increased hematocrit/hemoglobin (erythrocytosis).
- Suppression of spermatogenesis, testicular atrophy, infertility.
- Gynecomastia (via aromatization to estradiol).
- Changes in lipid profile (typically ↓HDL, variable effects on LDL and triglycerides).
- Worsening of benign prostatic hyperplasia (BPH) symptoms and increase in serum PSA.
Less common/serious adverse effects:
- Cardiovascular events: increased risk of hypertension, myocardial infarction, stroke, and other thromboembolic events in some patients — careful assessment of cardiovascular risk is recommended before and during therapy.
- Venous thromboembolism (deep vein thrombosis, pulmonary embolism).
- Polycythemia leading to hyperviscosity (can increase risk of thrombosis).
- Hepatic adverse effects: cholestasis or elevated LFTs are rare with injectable esters (more associated with 17α‑alkylated oral androgens), but LFT monitoring may be prudent in patients with liver disease.
- Severe psychiatric effects: mania, severe depression, hostility, or aggressive behavior.
- Allergic hypersensitivity reactions (rare).
- Potential worsening of sleep apnea.
- Rare: prostate cancer progression if preexisting — testosterone therapy is contraindicated in known prostate or male breast cancer.
Contraindications and cautions (summary):
- Known or suspected prostate or male breast cancer.
- Pregnancy and breastfeeding (testosterone causes virilization of female fetus).
- Uncontrolled severe heart failure, uncontrolled hypertension.
- Hematocrit elevated at baseline (risk of further erythrocytosis).
- Active thromboembolic disease.
When to seek urgent medical attention:
- Signs of thrombosis (sudden chest pain, shortness of breath, unilateral leg swelling), neurological deficits (possible stroke), severe jaundice, severe allergic reaction (rash, difficulty breathing, swelling), or significant behavioral changes.
5. Storage — How to store it
- Store at controlled room temperature, typically 20–25 °C (68–77 °F). Short excursions permitted per local product labeling (often 15–30 °C / 59–86 °F) — follow the product’s package insert for exact conditions.
- Protect from excessive heat and light. Do not freeze.
- Keep ampoules/syringes in original packaging until use to protect from light and contamination.
- Keep out of reach of children and pets.
- Do not use if the liquid is cloudy (unless the product label indicates otherwise) or contains particulate matter; for oil‑based testosterone, inspect for particulate contamination and discoloration — discard if abnormal and consult pharmacist/manufacturer.
- Dispose of used needles and syringes in approved sharps containers according to local regulations.
- Follow any additional storage/disposal instructions on the product leaflet.
Additional practical and safety points
- Testosterone therapy requires informed consent discussing benefits, risks, and alternatives, and should be prescribed by clinicians experienced in hormone management.
- Counsel patients about contraception and fertility: exogenous testosterone can severely impair fertility; patients desiring future fertility should discuss alternative strategies (e.g., gonadotropin therapy) with a specialist.
- Avoid sharing needles; use sterile technique to reduce infection risk (including HIV/HBV/HCV transmission).
- Review concomitant medications for potential interactions (e.g., anticoagulants may require closer monitoring; CYP3A4 inhibitors/inducers can alter testosterone metabolism).
Emergency/overdose
- Overdose is usually related to prolonged high dosing and leads to exaggerated androgenic effects and risks noted above (polycythemia, fluid retention, hepatic effects, behavioral changes). Management is supportive and symptomatic; seek urgent medical care for significant cardiovascular, thrombotic, hepatic, or psychiatric complications.
This guide is educational and not a substitute for individualized medical advice. For dosing adjustments, monitoring schedules, or concerns about adverse effects, patients should consult their prescribing clinician or a specialist in endocrinology.
1. Description
Test C 250 mg/mL (active compound: testosterone cypionate) is an oil-based injectable formulation of testosterone ester. It is a long-acting intramuscular androgen preparation used primarily for testosterone replacement therapy (TRT) in men with confirmed hypogonadism, and for masculinizing hormone therapy under specialist supervision. Testosterone cypionate is prescribed to restore physiologic serum testosterone levels, relieve symptoms of androgen deficiency (low libido, fatigue, decreased muscle mass, low mood), and maintain secondary sexual characteristics.
Formulation note: typical concentration is 250 mg/mL; dosing and injection volume should be calculated accordingly.
Indications (typical)
- Male hypogonadism (primary or secondary) confirmed by clinical features plus consistently low morning serum testosterone.
- Gender-affirming masculinizing hormone therapy (transgender men and non-binary people) under specialist care.
- Some approved or off-label uses under specialist supervision (e.g., certain cases of delayed puberty); not for enhancement in healthy individuals.
Contraindications (important)
- Known or suspected prostate or male breast cancer.
- Pregnancy (teratogenic risk) and breastfeeding.
- Uncontrolled severe heart failure, recent myocardial infarction or stroke (use with caution).
- Known hypersensitivity to testosterone or excipients.
2. How does testosterone-cypionate work?
Testosterone cypionate is a prodrug of testosterone. After intramuscular injection the ester is slowly hydrolyzed to release testosterone into the circulation, providing sustained serum levels over days to weeks.
Mechanisms:
- Binds to intracellular androgen receptors in target tissues. The hormone–receptor complex translocates to the nucleus and modulates transcription of androgen-responsive genes, producing anabolic (muscle, bone) and androgenic (secondary sexual characteristics, sexual function) effects.
- In target tissues, testosterone can be reduced to dihydrotestosterone (DHT) by 5α-reductase; DHT has higher androgen receptor affinity and mediates many androgenic effects (e.g., prostate growth, pattern hair loss).
- Testosterone is aromatized to estradiol by aromatase; estradiol mediates important effects on bone health and can contribute to gynecomastia and fluid retention.
Pharmacokinetics (practical points)
- Given intramuscularly as an oil suspension; not for intravenous use.
- Apparent elimination half-life after IM injection ≈ 6–8 days; clinically used for weekly to biweekly dosing.
- Steady-state and symptom improvement may take several weeks; endocrine effects are dose-dependent.
3. Dosage
Dosing should be individualized and prescribed by a clinician experienced in androgen therapy. The following are commonly used clinical regimens; always adjust based on clinical response and monitored serum testosterone concentrations and adverse effects.
General adult male replacement (typical ranges)
- Common: 50–100 mg intramuscularly every 1 week, or 100–200 mg every 2 weeks.
- Weekly dosing tends to produce more stable serum levels and fewer mood/energy fluctuations than large biweekly doses.
- Some prescribers use 75–100 mg weekly as a practical regimen.
- Dose adjustments guided by trough serum total testosterone (drawn just before the next injection), symptoms, hematocrit, PSA, and side effects.
Transgender masculinizing therapy
- Typical starting range: 50–100 mg IM every 1–2 weeks, titrated to clinical response and testosterone levels consistent with the target male physiologic range.
- Long-term protocols and monitoring should follow gender-affirming care guidelines.
Supraphysiologic (non-medical/anabolic) use
- Many nonmedical users take 200–1000 mg/week or higher. Such use carries significantly increased risk of adverse effects and is not medically recommended.
Special populations and cautions
- Older patients and those with comorbid cardiovascular disease: start at lower doses and monitor closely.
- Children/adolescents: use only under specialist supervision for specific indications (e.g., delayed puberty); dosing differs and requires expert pediatric/endocrinology oversight.
- Renal or hepatic impairment: use with caution; no well-established routine dose reductions but close monitoring is required.
- Fertility: replacement doses suppress LH/FSH and can reduce spermatogenesis; discuss fertility preservation prior to treatment if fertility is desired.
Administration technique (clinical points)
- Route: deep intramuscular injection (typically gluteal or lateral thigh/deltoid depending on volume and practitioner preference).
- Use an appropriate syringe and needle for IM oil injection (commonly 21–23 gauge; 1–1.5 inch needle for gluteal injections; adjust for body habitus).
- Rotate injection sites and observe standard aseptic technique. Avoid intravenous injection.
- Many clinicians prefer weekly injections to minimize peaks/troughs; if using biweekly dosing, be aware of greater variability in serum levels.
Monitoring and targets
- Baseline: total testosterone (morning), LH/FSH, CBC (hematocrit/hemoglobin), PSA (men >40 or per local guidance), lipid profile, liver function tests, blood pressure, and assessment of cardiovascular risk. Consider baseline bone density if indicated.
- After initiation: measure trough total testosterone 1–3 months after starting or dose change; aim for mid-normal physiologic range (varies by lab — often 300–1000 ng/dL in adult males; work with clinician to set goals).
- CBC (hematocrit): check at 3 months, 6 months, then every 6–12 months. If hematocrit rises above ~50–54%, consider dose reduction, temporary discontinuation, or therapeutic phlebotomy.
- PSA: baseline and periodic monitoring (e.g., at 3–6 months then according to urology guidance). Any unexplained PSA rise or urinary symptoms should prompt urology referral.
- Lipids and metabolic monitoring: check lipid profile periodically and manage cardiovascular risk factors.
- Monitor for signs of fluid retention, hypertension, mood/behavior changes, and symptoms suggesting thromboembolism.
Emergency and safety reminders
- In case of symptoms suggestive of thrombosis (sudden leg pain/swelling, chest pain, breathlessness), severe jaundice, severe mood changes or signs of prostate obstruction, seek immediate medical assessment.
4. Side effects
Testosterone therapy has a predictable adverse effect profile. Many adverse events are dose-dependent and more likely at supraphysiologic doses.
Common and relatively frequent effects
- Acne and oily skin.
- Increased libido (or changes in sexual function).
- Fluid retention and peripheral edema.
- Weight gain, increased muscle mass.
- Increased hematocrit/hemoglobin (erythrocytosis) — clinically important; may require dose adjustment or phlebotomy.
- Altered lipid profile — typically decrease in HDL cholesterol and variable effects on LDL/triglycerides.
- Suppression of spermatogenesis and decreased fertility (reversible months after discontinuation in many, but not always immediate).
- Local injection-site pain, swelling or irritation.
Less common but important effects / serious risks
- Prostate effects: may accelerate growth of occult prostate cancer and worsen benign prostatic hyperplasia; monitor PSA and lower urinary tract symptoms.
- Cardiovascular events: some evidence links testosterone therapy (especially in older men or those with preexisting cardiovascular disease) with increased risk of myocardial infarction, stroke, and venous thromboembolism in certain populations — data are mixed; discuss individualized risks with clinician.
- Psychiatric/behavioral: mood swings, irritability, aggression, mania or depression in susceptible individuals.
- Gynecomastia (due to aromatization to estradiol).
- Sleep apnea or worsening of existing sleep apnea.
- Cholestatic jaundice and hepatic dysfunction are less common with injectable testosterone esters than with 17α-alkylated oral androgens, but severe hepatic adverse reactions have been reported with androgens in general — monitor LFTs if clinically indicated.
- Allergic reactions: rare hypersensitivity to product or excipients.
- Rare: priapism (prolonged painful erection) — medical emergency.
Drug interactions and special considerations
- Anticoagulants (e.g., warfarin): androgens can alter coagulation and increase anticoagulant effects — monitor INR and adjust dosing as needed.
- Insulin and oral hypoglycemics: testosterone can alter glucose metabolism; monitor glycemic control.
- Corticosteroids: concurrent use increases risk of fluid retention and may increase edema.
- Inform patients about potential interactions with other medications and supplements.
Reproductive and developmental risks
- Women exposed during pregnancy can virilize a female fetus — absolute contraindication in pregnancy.
- Men should be counseled regarding potential impact on fertility; consider semen analysis and fertility preservation if relevant.
5. Storage
- Store at controlled room temperature, typically 20–25°C (68–77°F). Some formulations allow excursions 15–30°C (59–86°F); follow the product-specific package insert.
- Protect from light and excessive heat. Do not freeze.
- Keep the vial in its original carton (if provided) to protect from light.
- Keep out of reach of children and pets.
- Dispose of needles and syringes in an approved sharps container and follow local regulations for medical waste.
- Do not use if the solution is discolored, contains particulate matter, or the vial is damaged. Consult a pharmacist or prescriber if in doubt.
Final notes
- Testosterone cypionate is a prescription medication and should be used only under medical supervision with appropriate baseline assessment, ongoing monitoring, and awareness of contraindications and potential adverse effects.
- If you have specific clinical questions about dosing for a particular patient, monitoring interpretation, or managing adverse effects, discuss with an endocrinologist, urologist, or other qualified prescriber. In an emergency (suspected thrombotic event, severe allergic reaction, jaundice, priapism, chest pain), seek immediate medical attention.
1. Description
Test C 250 mg/mL (active compound: testosterone cypionate) is an injectable, oil-based depot formulation of testosterone. It is an esterified form of testosterone designed for intramuscular (IM) administration that provides a slow, sustained release of active testosterone into the circulation. Clinically it is used primarily for testosterone replacement therapy (TRT) in males with hypogonadism (congenital or acquired), for induction/maintenance of puberty in selected adolescents under specialist care, and as part of masculinizing hormone therapy for transgender men. Testosterone cypionate is a prescription controlled substance (Schedule II in the U.S.) and should be used only under medical supervision.
Key points:
- Concentration commonly: 250 mg per mL (oil solution).
- Route: deep intramuscular injection.
- Intended effect: restore/maintain physiologic androgen levels and androgenic/anabolic effects.
2. How does testosterone-cypionate work?
- Prodrug and release: Testosterone cypionate is an esterified testosterone molecule. After IM injection, the ester is slowly hydrolyzed by tissue and plasma esterases to release active testosterone over days to weeks.
- Androgen receptor activation: Testosterone binds androgen receptors in target tissues (muscle, bone, reproductive organs, skin, brain), modulating gene expression to produce androgenic and anabolic effects (e.g., increased protein synthesis, muscle mass, libido, virilization).
- Peripheral metabolism: Testosterone is converted peripherally to dihydrotestosterone (DHT) by 5α-reductase (more potent androgenic activity in some tissues) and to estradiol by aromatase (mediating estrogen-related effects such as bone maintenance and potential gynecomastia).
- Endocrine feedback: Exogenous testosterone suppresses the hypothalamic–pituitary–gonadal axis (↓ GnRH → ↓ LH/FSH), which reduces endogenous testicular testosterone production and sperm production (can cause oligo/azoospermia and testicular atrophy).
- Systemic effects: Androgens increase erythropoiesis (risk of polycythemia), can alter lipid profiles, influence fluid balance, and affect cardiovascular and metabolic parameters.
3. Dosage
Dosage must be individualized by a qualified clinician based on indication, patient age, body size, baseline hormone levels, comorbidities, and monitoring results. The following are general guidance ranges frequently used in clinical practice.
General administration:
- Route: Deep intramuscular injection (gluteal muscle is common for adults). Use aseptic technique; change needle after drawing from vial if necessary; avoid intravenous injection.
- Concentration reference: 250 mg/mL — a 1 mL injection delivers 250 mg testosterone cypionate.
- Typical needle: 22–23 gauge, 1–1.5 inch for gluteal IM in adults (adjust by body habitus).
Common medically accepted dosing regimens (adults with hypogonadism and transgender men):
- Typical TRT regimens:
- 50–100 mg IM once weekly, or
- 100–200 mg IM every 1–2 weeks, or
- Some clinicians use 200–400 mg IM every 2–4 weeks (larger intervals produce greater peak–trough fluctuations).
- Aim: achieve and maintain serum total testosterone in the mid-normal physiologic range for young adult males (laboratory reference range varies; commonly ~300–1000 ng/dL). Measure levels mid-interval (e.g., 1 week after injection for biweekly dosing or 48–72 hours for weekly dosing) to guide dose adjustments.
- For transgender masculinizing therapy: many protocols use 50–100 mg IM every 7–14 days or 100–200 mg every 2 weeks; dose titrated to clinical effect and target testosterone range.
Adolescents (induction of puberty) and elderly:
- Adolescents: Start with low doses and titrate slowly under specialist care (e.g., small monthly doses in early induction, supervised escalation).
- Elderly or frail patients: Start at lower end and monitor closely for cardiovascular and prostate effects.
Monitoring and dose adjustment:
- Baseline tests: serum testosterone, LH/FSH, hematocrit/hemoglobin, PSA (men >40 or per guidelines), liver function tests, lipid profile, and cardiovascular risk assessment.
- Ongoing monitoring: testosterone level after steady state (2–3 weeks after start or dose change), hematocrit/Hb every 3 months during first year then periodically, PSA and digital rectal exam per guidelines (usually baseline and periodically thereafter), lipids and LFTs as indicated.
- Adjust dose to keep testosterone in desired range and to avoid supraphysiologic peaks.
- If fertility is desired, discuss sperm preservation before starting therapy because exogenous testosterone commonly suppresses spermatogenesis.
Non-medical/high-dose use:
- Illicit or supraphysiologic dosing (commonly seen in anabolic steroid misuse) frequently involves much higher doses (e.g., 200–1000+ mg/week); this markedly increases risk of severe adverse events and is strongly discouraged. Provide counseling rather than dosing recommendations for such use.
Contraindications and cautions:
- Absolute contraindications: known or suspected breast cancer or prostate cancer in men; pregnancy (teratogenic to a female fetus); hypersensitivity to components.
- Use with caution in: severe cardiovascular disease, uncontrolled hypertension, severe hepatic disease, polycythemia (hematocrit >50%), sleep apnea, severe benign prostatic hyperplasia.
- Drug interactions: may potentiate anticoagulants (warfarin), impact insulin/antidiabetic drugs, and interact with corticosteroids (edema risk).
Overdose:
- Acute overdose is unlikely to be life-threatening; symptoms may include nausea, vomiting, virilization (in females/children), fluid retention, and cardiovascular symptoms. Management is supportive; seek medical care.
4. Side effects
Adverse effects range from common and generally manageable to rare but serious. Risk increases with dose and duration, and with supraphysiologic use.
Common and dose-related (frequent):
- Injection site reactions: pain, redness, transient swelling.
- Androgenic effects: acne, oily skin, increased facial/body hair, male-pattern scalp hair loss.
- Fluid retention and weight gain.
- Increased hematocrit/hemoglobin (polycythemia), which raises thrombotic risk.
- Suppression of spermatogenesis, testicular atrophy, reduced fertility.
- Mood changes: irritability, mood swings, changes in libido (increase or, paradoxically, decrease).
- Gynecomastia (due to aromatization to estradiol).
- Changes in lipid profile: typically decreased HDL, possible increase in LDL (atherogenic shift).
- Sleep apnea may worsen or be unmasked.
Less common / potentially serious:
- Cardiovascular events: increased risk of hypertension, myocardial infarction, stroke in some patient populations—data mixed; caution in patients with cardiovascular disease.
- Venous thromboembolism (deep vein thrombosis, pulmonary embolism), particularly in the setting of polycythemia or other risk factors.
- Prostate effects: benign prostatic hyperplasia exacerbation, increased PSA; screening/monitoring for prostate cancer per guidelines is recommended (testosterone does not cause prostate cancer but can stimulate growth of existing disease).
- Liver abnormalities: serious hepatic toxicity is uncommon with injectable testosterone (more associated with 17α-alkylated oral androgens), but monitor LFTs when clinically indicated.
- Rare allergic or hypersensitivity reactions.
Pediatrics and pregnancy:
- Exposure in utero or in women of childbearing potential may cause virilization of a female fetus; contraindicated in pregnancy.
Reporting and management:
- Monitor hematocrit and stop/adjust therapy if hematocrit exceeds recommended limits (commonly >54%).
- Manage gynecomastia by dose reduction, aromatase inhibitor (specialist decision), or surgical options if persistent.
- For severe psychiatric effects or cardiovascular symptoms, stop therapy and seek specialist evaluation.
- Counsel on contraception and fertility implications.
5. Storage
- Store at controlled room temperature: typically 20–25°C (68–77°F). Brief excursions permitted within manufacturer guidelines (often 15–30°C). Consult the product’s official prescribing information for exact temperature range.
- Protect from excessive heat and direct sunlight. Do not freeze. If stored refrigerated by error, allow vial to return to room temperature before use.
- Keep the vial in its original container to protect from light.
- Maintain sterility: use aseptic technique when withdrawing doses. Single-dose vials should not be reused. For multi-dose vials follow manufacturer guidance and local infection-control policies; discard if contamination or if beyond recommended time after first puncture.
- Keep out of reach of children and pets.
- Dispose of used syringes, needles, and vials in approved sharps containers per local regulations.
Final notes:
- Testosterone cypionate therapy should be initiated and followed by a clinician experienced in hormone therapy. Regular monitoring (testosterone levels, hematocrit, PSA where appropriate, lipids, LFTs, clinical symptoms) and individualized dosing are essential to maximize benefit and reduce risk.
- This guide summarizes common clinical practice and is not a substitute for professional medical advice tailored to an individual patient.